SiliaBond Reagents
SiliaBond Reagents
| SiliaBond Reagents |
SiliaBond Reagents
Amide Coupling Reagents
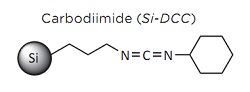
The amide bond is the defining molecular structure of proteins and peptides. One in addition, a report estimates that as many as 25% of all synthetic pharmaceutical drugs contain an amide group.Therefore, there is an ongoing scientific endeavor to develop efficient amidation methodologies Usually, the amide bond formation relies on the use of an excess of toxic coupling reagents such as carbodiimides or supernucleophiles. These chemicals produce a large amount of by-products, which tends to complicate the isolation and purification of the desired amide product. The use of a reagent linked to an insoluble material has become a widely used tool since the introduction of the solid-phase synthesis concept.3 Solid-phase reagents are valuable for amide coupling with a carboxylic acid because of the lack of unwanted side products. Other advantages to using solid supported reagents include improved stability, toxic chemical immobilization, the ability to run multiple transformations in a single pot, and the flexibility to use both batch reactions and flow chemistry.
SiliaBond Cyanoborohydride for Reductive Amination
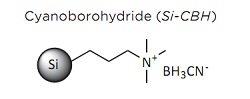
Reductive amination involves the conversion of a carbonyl group, most of the time a ketone or an aldehyde, to an amine by an intermediate imine or iminium. The intermediate imine is reduced by sodium cyanoborohydride. This is known as direct reductive amination, and is carried out with reducing agents that are more reactive toward protonated imines than ketones and are stable under moderately acidic conditions.
SiliaBond Carbonate for Henry Reaction
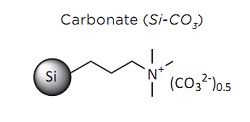
The Henry reaction is commonly used to form carbon-carbon bonds by addition of nitroalkanes over aldehydes. This reaction is a useful technique in organic chemistry due to the synthetic utility of its corresponding products, as they can be easily converted to other useful synthetic intermediates such as nitroalkenes by dehydrogenation, alphanitro ketone by oxidation and ß-amino alcohol by reduction. Usually, the Henry reaction is carried out in presence of bases in homogeneous solution, giving low yield due to side reactions such as retroaldol and Cannizarro reactions
SiliaBond DMAP for Baylis-Hillman and Acylation Reactions
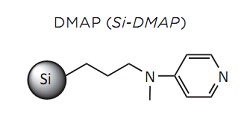
Baylis-Hillman Reaction Coupling of activated alkenes, generally alpha, 1-beta-unsaturated, with aldehydes is named the Baylis-Hillman reaction. This reaction is well known for the formation of carbon-carbon bonds under soft conditions and its compatibility with several functional groups. Furthermore, an organic base can be used to catalyze this reaction with similar success to using transition metals.
SiliaBond Tosic Acid in Fischer-Speier Esterification
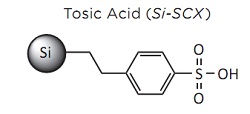
The Fischer-Speier reaction is a classic organic process where a carboxylic acid is reacted with an alcohol in the presence of an acidic catalyst to form an ester. All carboxylic acids and only primary and secondary alphatic alcohols can be use in this reaction. The most commonly used catalysts for this reaction are highly toxic such as H2SO4, tosic acid and candium triflate. Also, a large excess of one of the reagents is used to push the equilibrium towards the product.
SiliaBond TBD for Williamson Etherification
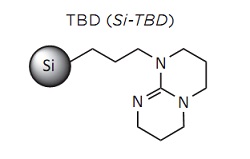
Williamson etherification is standard reaction to synthesize asymmetric ethers from alcoholates, prepared from primary and secondary alcohols or phenols with base, in the presence of primary alkyl halides. Because of the high reactivity of alcoholates, they need to be produced during the reaction by strong bases.
SiliaBond Aluminum Chloride Used as a Catalyst for Friedel- Crafts Alkylation and Acylation
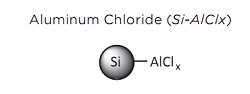
For decades, sulfonated linear alkylbenzenes (LABs) have been among the most prolific detergents. LAB synthesis is carried out by Friedel-Crafts alkylation of benzene by linear olefins using hydrogen fluoride or aluminum chloride as catalyst. The use of these catalysts presents severe problems, however. For example, aluminum chloride is difficult to separate after reaction and produces a large amount of waste effluent.

